Our experience
We serve as acting CMO, yet also provide extended stand-alone
as well as fully-integrated consulting services to the biotechnology and pharmaceutical industry,
with a particular focus on rare and orphan diseases, oncology, as well as biotherapeutics and advances therapies.
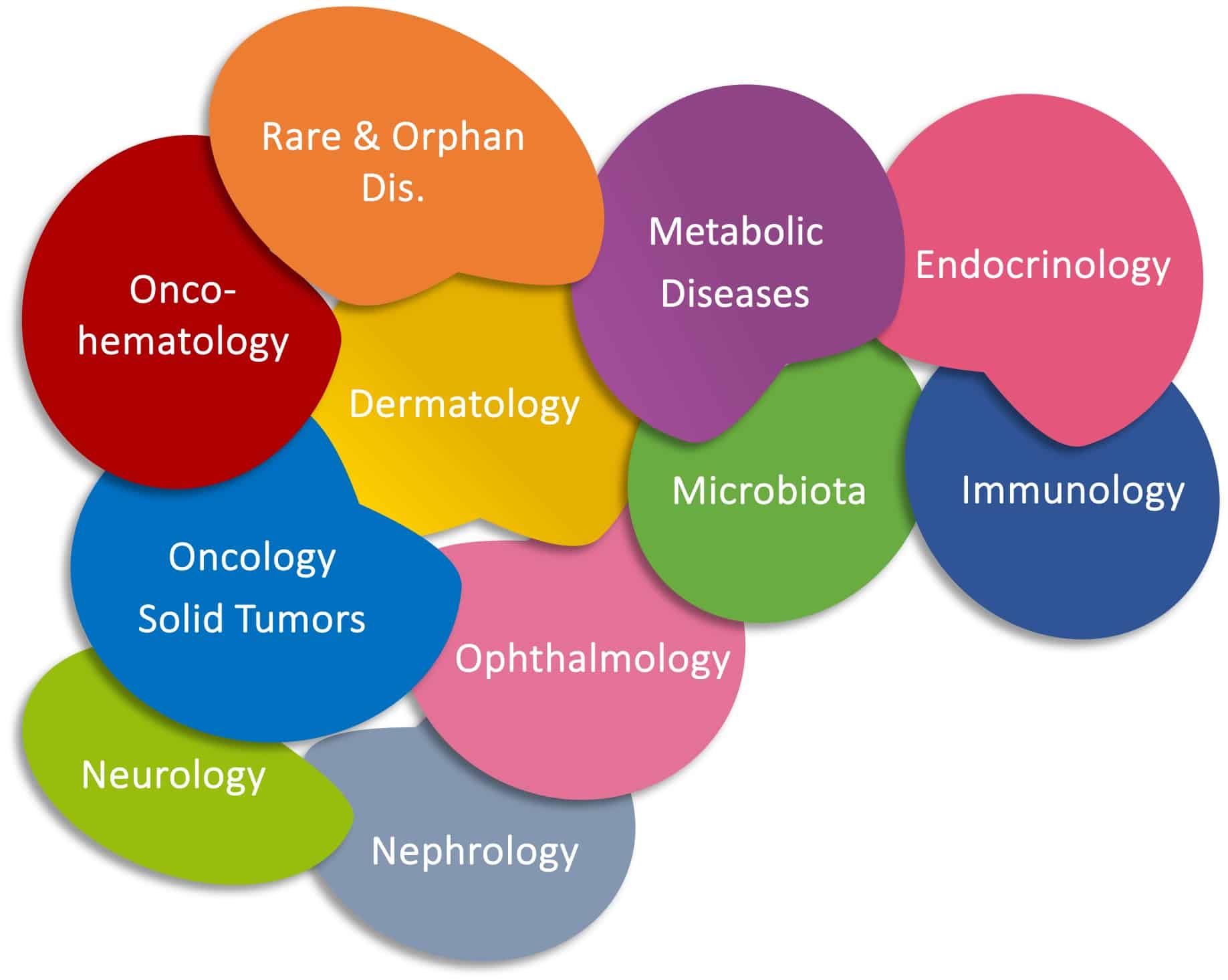

Therapeutic areas
Mucopolysaccharidosis (MPS)
Neuromuscular disorders
Peripheral Neuropathies
Genetic nephropathies
Primary immune deficiencies
Oncology
Ono-hematological diseases
Endocrine diseases
(Graves' orbitopathy)
Activities
Clinical development plans
Protocol design
KOLs & Experts management
Medical review & medical writing
Site identification
Clinical study feasibility
Regulatory submissions
Phase Ib-II-III studies set-up & monitoring
Non-interventional studies
Scientific and medical support for patient enrollment
Patient Support & Patient Access Programs
Medical Affairs plans drafting and strategy execution
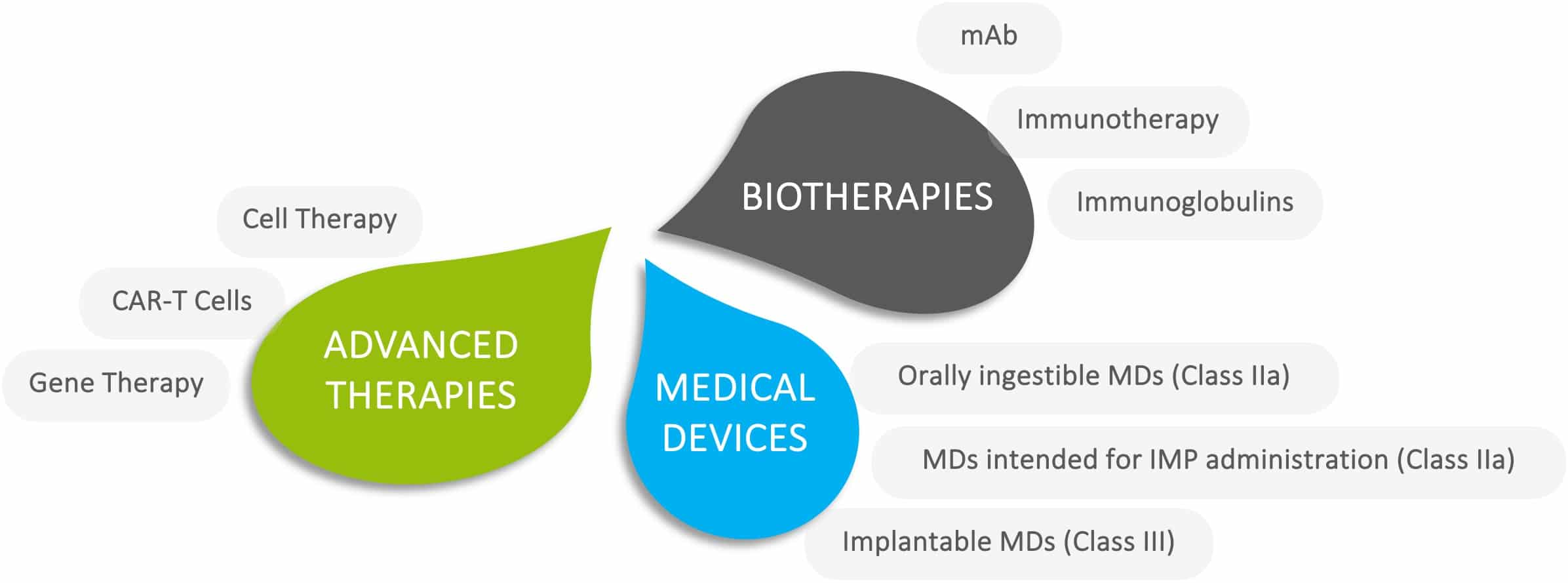
Products
Gene therapy
Cell therapy
CAR-T cell therapy
IVIg
Immunotherapy
Therapeutic peptides
Enteric biotherapy
Small molecule repositioning
Medical devices
Business Cases
Site visits
Scientific and medical communication of investigators
Identification of hurdles to enrollment and proposal of action plan
Therapeutic area: Hematology / Microbiota
70 sites, 12 European countries
Feasibility study
Set up, management & monitoring of a phase III + extension studies
Therapeutic area: Rare genetic nephropathy (pediatric)
Product: Enteric biotherapy
Tunisia, 3 sites, 10 patients
KOLs & expert identification & management
Synopsis design & drafting (2 phase II studies)
Therapeutic area: Endocrinology/ auto-immune diseases
Product: Immunotherapy
Germany, Italy, The Netherlands, Turkey
Interaction with regulatory authorities
Set up of clinical trials
Therapeutic area: Onco-hematology
Product: CAR-T cell therapy
50 sites, 55 patients
20 countries, including Europe, UK, USA, Brazil, Australia, South Korea
Clinical protocol drafting (Phase IIa / phase Ib / Phase III)
KOLs & experts management
Set up, conduct & medical monitoring of clinical studies (phase IIa and Ib)
Scientific & medical support for enrollment
Interaction with regulatory authorities
Therapeutic area: Metabolic disease (rare genetic disease, pediatric)
Product: small molecule (repurposing)
4 sites, 24+9 patients
France Germany, Portugal, UK
Interaction with regulatory authorities
Therapeutic area: Infectious diseases
Product: vaccine
3 sites, 600 patients
Morocco
Interaction with regulatory authorities
First-in-human clinical study set up
Therapeutic area: Oncology (solid tumors)
Product: therapeutic peptide
2 sites
Europe
Protocol amendments drafting
Interaction with regulatory authorities
Medical review
Therapeutic area: Onco-hematology
Product: Monoclonal antibody
145 sites, 21 countries, 500 patients
Europe, UK, USA, South America, Australia, Japan, China, Taiwan